
Symbol glossary
Our packaging design and labeling meets international standards and regulations. We have also adopted and developed symbols that simplify communication regarding product features for example, all are compiled below including the meaning of the symbols.
Medical device
Indicates that the device is a medical device as defined in MDR 2017/745-
Manufacturer
Indicates the medical device manufacturer, as defined in EU Directives 90/385/EEC, 93/42/EEC and 98/79/EC. SS-EN ISO 15223-1:2021
Date of manufacture
Indicates the date when the medical device was manufactured
Use-by date
Indicates the date after which the medical device is not to be used
Batch code
Indicates the manufacturer’s batch code so that the batch or lot can be identified. SS-EN ISO 15223-1:2021
Catalogue number
Indicates the manufacturer’s catalogue number so that the medical device can be identified
Sterilized using ethylene oxide
Indicates a medical device that has been sterilized using ethylene oxide
Sterilized using irradiation
Indicates a medical device that has been sterilized using irradiation
Single use
Indicates a medical device that is intended for one single use only
Single sterile barrier system
Indicates a single sterile barrier system
Single sterile barrier system with protective packaging inside
Indicates a single sterile barrier system with protective packaging inside
Non-sterile
Indicates a medical device that has not been subjected to a sterilization process
Drops per millilitre
Indicates the number of drops per millilitre
Fluid path
Indicates the presence of a fluid path
One-way valve
Indicates a medical device with a valve that allows flow in only one direction
Non-pyrogenic
Indicated a medical device that is non-pyrogenic
Liquid filter with pore size
Indicates an infusion or transfusion system of the medical device that contains a filter of a particular nominal pore size
Caution
Indicates that caution is necessary when operating the device or control close to where the symbol is placed, or that the current situation needs operator awareness or operator action in order to avoid undesirable consequences
Consult instruction for use
Indicates the need for the user to consult the instructions for use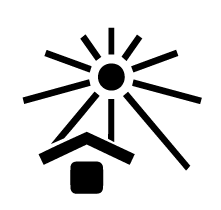
Keep away from sunlight
Indicates a medical device that needs protection from light sources
Keep dry
Indicates a medical device that needs to be protected from moisture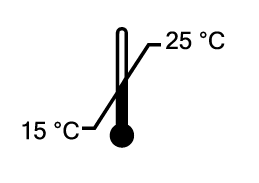
Temperature limit
Indicates the temperature limits to which the medical device can be safely exposed. *The temperature shown in the symbol is assested by OMP QE team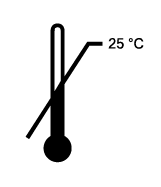
Upper limit of temperature
Indicates the temperature limits to which the medical device can be safely exposed. *The temperature shown in the symbol is assested by OMP QE team
Do not use if package is damaged and consult instructions for use
Indicates that a medical device that should not be used if the package has been damaged or opened and that the user should consult the instructions for use for additional information
Do not open with knife
Symbol indicates that the user should not use a knife for opening the package due to the risk of damaging the medical device inside
Does not contain or there is no presence of natural rubber latex
Symbol indicates that the device does not contain or that there is no presence of natural rubber latex
Product does not DEHP
Symbol indicates that the device does not contain DEHP
Product does not PVC
Symbol indicates that the device does not contain PVC
4 ply
Indicates the number of plyes the swab has
8 ply
Indicates the number of plyes the swab has
12 ply
Indicates the number of plyes the swab has
3-way stopcock
Indicates a medical device that is a 3-way stopcock or has a 3-way stopcock component
Tube lenght
Indicates that the medical device includes tubing
Safe for use with pressure infusion equipment
Indicates that the medical device is safe or intended for use with pressure infusion equipment
Gravity feed only
Indicates that the medical device should only be used for gravity feed and that the device IS NOT safe or intended for use with pressure infusion equipment
Luer lock
Indicates a medical device that has a luer lock component
Needle free connector
Indicates a medical device that has a needle free connector component
Mask with Anti Reflection and Ear-loop
Symbol indicates that the mask has anti reflection properties and ear-loops
Mask with Anti Reflection and Tie-band
Symbol indicates that the mask has anti reflection properties and tie-band
Anti-fog properties
Symbol indicates that the mask has anti reflection properties
Tie-band
Symbol indicates that the mask has tie-band
Ear-loop
Symbol indicates that the mask has ear-loop
Extra wide
Symbol indicates that the mask is wider then the rest of the mask
Soft
Symbol indicates that the mask is made of softer material or that the material the mask is made of has soft properties then the rest of the mask
Splash resistant
Symbol indicates that the mask has splash resistant properties
Visor
Symbol indicates that the mask has visor
Latex free
Symbol indicates the composition of device not containing natural rubber latex or that its non-presence of is confirmed by a test
Basic procedures and wards
Recommendation for use in basic procedures and wards
Critical care
Recommendation for use in Critical care
Pharmacy
Recommendation for use in Pharmacy
Medical laboratory
Recommendation for use in Medical laboratory
Good protection against infection
Recommendation for use in more critical procedures and contact with blood
Sampling
Recommendation for use in sampling and injection work
Dental care
Recommendation for use in Dental care
Cleaning and surface disinfection
FSC certification ensures that products come from responsibly managed forests that provide environmental, social and economic benefits
Basic cleaning
Recommendation for use in Basic cleaning
Hairdressing
Recommendation for use in Hairdressing
Baby care
Recommendation for use in Baby care
Recycle symbol
Package is recyclable
Food safe
Suitable for handling food. If there are any restrictions they need to be beside the symbol. The restrictions need also to be translated
Micro-organisms and virus hazards
Protective gloves against dangerous chemicals and micro-organisms — Part 5: Terminology and performance requirements for micro-organisms risks
Flammable
Warning symbol for flammable substances, needs to be explained with additional text
Exclamation Mark
Warning symbol needs to be explained with additional text
Period after opening symbol
Symbol should also have the according nr of months writtenCE-mark
Indicates manufacturer declaration that the product complies with the essential/ general safety & performance requirements of the relevant European medical device, health, safety and environmental protection legislations.European Medical Devices Directive 93/42/EEC of 14 June 1993 (as amended by Directive 2007/47/EC). European Medical Device Regulation 2017/745

CE-mark with notified body number
Indicates manufacturer declaration that the product complies with the essential/ general safety & performance requirements of the relevant European medical device, health, safety and environmental protection legislations.European Medical Devices Directive 93/42/EEC of 14 June 1993 (as amended by Directive 2007/47/EC). European Medical Device Regulation 2017/745
Wash
Recommendation for use when washing patients
Food handling
Recommendation for use when handling food
Recycle symbol for corrugated fiberboard
Symbol indicates packaging is recyclable
Recycle symbol for non-corrugated fibreboard
Symbol indicates packaging is recyclable.
Protection against micro-organisms
Protective gloves against bacteria and fungi or against viruses, bacteria and fungi
Protection against dangerous chemicals
Protective glove against dangerous chemicals and micro-organisms complying with Type A, Type B or Type C requirements stated in standard EN ISO 374-1
Sterile
Indicates a medical device that has been subjected to a sterilization process
Do not resterilize
Indicates a medical device that is not to be re-sterilized
Contains or presence of natural rubber latex
Indicates the presence of dry natural rubber or natural rubber latex as a material of construction within the medical device or the packaging of medical device
Unique device identifier
Indicates a carrier that contains uniques device identifier information
Importer
Indicates the entity importing the medical device into the locale
Distributor
Indicates the entity distributing the medical device into the locale
Authorized representative in the European Community / European Union
Indicates the authorized representative in the European Community/ European Union
Health care professional use
Symbol indicates that the device is intended to be used by health care professionals






